IAP voice on regenerative medicine
Regenerative medicine has great potential for tissue regeneration and repair, comprising various novel interdisciplinary approaches including the use of cell and gene therapies, and tissue engineering. The pace of advance in this area is exciting, and the medical opportunities in addressing the causes of disease rather than the symptoms may be transformative, but concerns about the misuse of regenerative medicine technologies also grow. The InterAcademy Partnership network has issued a statement on this matter.
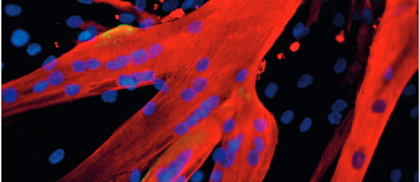
Human stem cells from ongoing research by Prof. Giulia Cossu (University of Manchester) on stem-cell therapy for muscular dystrophy.
The InterAcademy Partnership (IAP) is a global network of more than 140 national academies of science. It brings together more than 30,000 leading scientists, engineers and healthcare professionals in over 100 countries.
In this consensus Statement the InterAcademy Partnership (IAP) seeks to raise awareness of two main priorities:
- To use advances in research and development as rapidly as possible, safely and equitably, to provide new routes to patient benefit.
- To support medical claims by robust and replicable evidenceso that patients and the public are not misled.
What is regenerative medicine?
Regenerative medicine comprises a variety of cutting-edge interdisciplinary approaches that use the body’s own ability to heal. It is the study of the regeneration mechanisms at the cellular and molecular level.
The IAP's statement, based on the work of academia-nominated experts from Africa, Asia, the Americas and Europe, also serves to integrate and strengthen the voice of low and middle-income countries (LMICs) whose positions are sometimes ignored. Recommendations for fostering research and innovation while protecting patients include ethical review, pre-clinical and clinical research phases, regulatory authorization and access to new drugs, and the involvement of patients, policy makers and the public.
Volker ter Meulen, special adviser to the IAP and chairman of the working group that developed the statement, stressed that “accelerated access is a vital tool for patient benefit, but researchers must not cut corners. Therefore, to deliver clinical benefits equitably, a coordinated strategy must encompass better science, better funding, better governance and better public and patient engagement”.
The IAP position focuses on unmet medical needs: stem cells are an example of a case study with many conclusions on the broader regenerative medicine. While stem cell therapy is well established for only a limited number of clinical indications, such as bone marrow or epidermal transplantation, active research and development is underway in many others, including neurological, metabolic, cardiovascular disorders, visual disturbances, muscular and systemic disorders skeletal.
The focus of the IAP Statement is on unmet medical needs: stem cells are an example of a case study with many conclusions relevant more broadly for regenerative medicine. Although, stem cell therapies are well-established in only a limited number of clinical indications, such as bone marrow or epidermis transplantation, in congenital immunodeficiency, and lysosomal storage disease, active research and development is underway for many others, including retinal, neurological, hepatic, cardiovascular and musculoskeletal disorders.
However, enthusiasm about the clinical potential has led to a disconnect between expectations and the realities of translating advances in technology into clinical practice. In many countries, there are two main problems. First, unscrupulous private clinics offer unregulated therapies that promise much but use poorly characterized products with little scientific basis or evidence for efficacy and safety. Second, premature regulatory approval and commercialization based on some, but insufficient, scientific rationale and clinical evidence.
Regenerative Medicine and COVID-19
An example of opportunities and challenges related to the use of stem cells are responses to the COVID-19 pandemic.
“Any such use must be based on rigorous evidence of safety and efficacy, following strict research protocols that consider the ethical issues and characterise the stem cells used, focusing on a defined stage of the disease and in the hands of a team with capacity and validity to undertake the intervention” – says Depei Liu, IAP Vice-President.
Unfortunately, as the FDA observes, some of the same clinics in the USA that have been offering unproven regenerative medicine therapies for diverse conditions are now offering unproven treatments for the treatment of complications of COVID-19. While some research is in progress, e.g. on mesenchymal stromal cells, the preliminary studies are insufficient to support commercialization.
There is a further concern regarding these unproven treatments for COVID-19: fraudulent claims of efficacy may encourage purchasers to abstain from taking other steps, e.g. social distancing, to protect themselves and others from COVID-19, or obtain an available COVID-19 vaccine.
"While there may be scientific rationale to discover and pursue new research lines in response to COVID-19, premature use of untested approaches jeopardizes patient safety and undermines public confidence," – warns IAP co-chair Sir Richard Catlow.
The scope of regenerative medicine includes:
- Cell transplantation, where cells originate from human embryonic stem cells, perinatal stem cells, induced pluripotent stem cells or tissue specific (adult) stem cells or other forms of cell therapy
- Gene therapy, both in vivo and ex vivo, the latter being a form of cell therapy
- Tissue engineering, typically using 3D scaffolds formed from either natural biomaterials or artificial, biocompatible biomaterials produced from a variety of fabrication processes
- Organoids, from adult and pluripotent stem cells
- Small-molecule drugs
- Subcellular bodies (e.g. mitochondria, vesicles)
- Artificial cells (currently prokaryotic only) and other synthetic biology approaches
Regenerative medicine strategies depend upon harnessing, stimulating, guiding or replacing endogenous development and repair processes.
We encourage you to read the full text of the IAP Statement on Regenerative Medicine.
