First observation of Pauli crystals
The theory of scientists from the PAS Institute of Physics has been experimentally confirmed by German physicists from the University of Heidelberg. The experiment concerns the formation of Pauli crystals – the structures in which atoms can arrange themselves in an unusual geometric configurations. This is another step towards the development of quantum technologies that will change our lives.
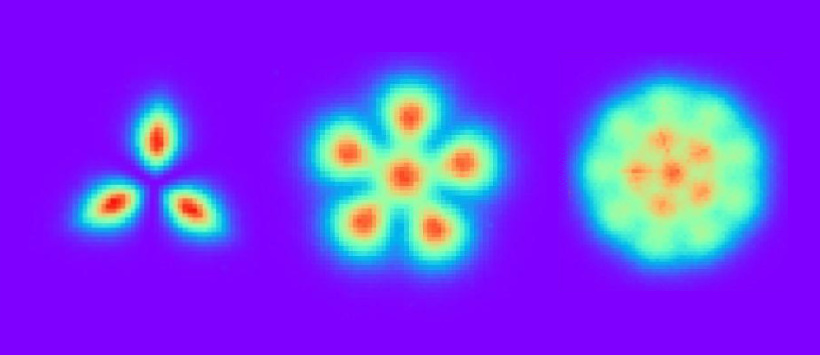
Probable configurations of Pauli crystals corresponding to the results of measurements performed for group of three, six, and fifteen atoms
In crystals (e.g. salt, sugar or diamond), the atoms form a regular network structure. This is possible thanks to the forces acting between them (chemical bonds are formed). In Pauli crystals, atoms form regular shapes (crystal-like arrangements) even though they do not exert any forces on one another.
This phenomenon occurs when the diluted gas is cooled to a temperature close to absolute zero, and appropriate magnetic field, in which atoms do not interact with each other, is used. Under these unique circumstance atoms spontaneously arrange into geometric structures. This surprising observation is a manifestation of the laws of quantum physics to which microscale matter is subject.
The scientific basis of the phenomenon
Atoms organize themselves into a specific shape due to the Pauli exclusion principle - one of the most non-intuitive quantum laws. This rule only applies to particles called fermions. In the case of neutral atoms, fermions are those with an odd number of neutrons.
The rule is that no two identical fermions can occupy the same quantum state. To put it simply, it can be said that fermions behave like conflicting siblings who do not want to live in one room and insist on single accommodation.
In 2015 – 90 years after Wolfgang Pauli formed his exclusion principle – scientists from the Institute of Physics of the Polish Academy of Sciences in Warsaw have theoretically demonstrated that the consequence of the rule is the unusual arrangement of atoms in space. The Polish team of scientists that developed the concept of so-called Pauli crystals and determined the geometric forms of these objects include Prof. prof. Jan Mostowski, prof. Mariusz Gajda, prof. Tomasz Sowiński and prof. Magdalena Załuska-Kotur.
Recently a research group headed by Prof. Selim Jochim from the University of Heidelberg has confirmed this theory by the experiment with lithium atoms. German physicists have succeeded in creating apparatus that allowed them to observe Pauli crystals for the first time. Their paper has been submitted to the arXiv preprint server.

Authors of Pauli crystals theory. From left: Prof. Tomasz Sowiński, Prof. Mariusz Gajda, Prof. Magdalena Załuska-Kotur and Prof. Jan Mostowski
Quantum technology boom is coming
Observing Pauli crystals is another step to a better understanding of the laws of physics, but above all, to the development of quantum technologies that will change our daily lives. Quantum technologies, which use the properties of quantum effects, have brought major technical advances in many different areas, including computing, sensors, simulations, cryptography and telecommunications. For instance, quantum computers will soon outperform traditional computers and other electronic devices with their processing capabilities. Thanks to quantum technologies the MRI scanners will be smaller (the size of smartphones) and much faster than those we know today.
Source of information and photos: PAS Institute of Physics
